Why?
Li-S batteries are promoted as a highly promising energy storage solution, but the specific energy demonstrated so far (~400 W h kg‑1) is restricted by the low utilization of active materials in both lithium and sulfur electrodes. The incomplete discharge process of the sulfur electrode is associated with precipitation of insulating reaction products from polysulfide intermediates. This process, however, is still largely unclear, but essential to improve the cell chemistry and structure. Here, we provide a mechanistic understanding of these multiphase reactions (see Fig. 1) in an operating battery through a combination of scattering measurements, including small and wide angle scattering with neutrons and x-rays. Since these use different contrasts, we can identify not only the crystalline solids, but also the amorphous product and the compositional variation of the electrolyte. Through simultaneously measured cell resistance, we can target the limitations of the discharge capacity in these electrochemical cells, and thereby render insights to overcome these bottlenecks.
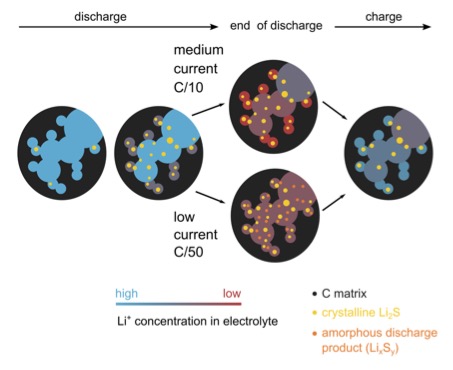
Figure 1. An illustration of the different precipitation reactions in the porous sulfur electrode during battery operation, and their dependence on current strength.
How?
Through a combination of scattering measurements using different contrasts – small and wide-angle scattering with neutrons and X-rays, performed at Institute Laue-Langevin (ILL)and Uppsala University, respectively – this work identifies not only the crystalline solids, but also the amorphous product and the compositional variation of the electrolyte in the porous electrode during battery operation. With simultaneously measured cell resistance by means of electrochemistry, limitations of the discharge capacity are identified to guide further development. The results suggest that Li+ transport stops the discharge at medium current.
What´s next?
Considering that the work highlights the bottlenecks in the sulfur electrode, the challenge now is to combine cell design and battery utilization to increase the sulfur utilization and thereby improve battery performance. Similar operando scattering experiment, but for a range of different porous electrode structures, will render improved insights into this complex electrochemical system.
Who?
The work resulted from an internal collaboration between Daniel Brandell (Ångström Advanced Battery Centre, Uppsala University) and Matthew Lacey (previously at Uppsala University, now at Scania), who has run research projects together on Li-S batteries for 10 years, and Adrian Rennie (Uppsala University), who has extensive experience with scattering techniques. The work was primarily carried out by PhD Yu-Chuan Chien at Uppsala (now at Breathe Battery Technologies), with assistance from Nina-Juliane Steinke at the ILL.
Cite: Chein et al., Correlations between Precipitation Reactions and Electrochemical Performance of Lithium–Sulfur Batteries Probed by Operando Scattering Techniques, Chem, 2022
For the original articles, please visit: https://doi.org/10.1016/j.chempr.2022.03.001
Contact:
Prof. Daniel Brandell
Ångström Advanced Battery Centre
Uppsala UniversityE-mail: Daniel.Brandell@kemi.uu.se
