Why?
Ionic liquids (ILs) have been identified as promising replacements for conventional lubricants. Their ionic nature and wide electrochemical windows present a new horizon for electrical energy storage solutions and tribotronic lubrication, whereby the friction coefficient is controllable though use of an electric field. However, due to the high production costs the commercialisation of IL lubricants has been largely limited. Furthermore, most of the ILs which have been studied for their tribological properties typically have contained F-containing anions (e.g. BF6 and PF6), whose highly polar and hygroscopic nature has limited their use as oil additives and prevented their application in tribological systems, where under high pressures and temperatures, they can react to produce toxic and corrosive hydrogen halides. Therefore, the development of more hydrophobic, non-halogenated ILs which can act as lubricant additives in ambient conditions is highly desirable.
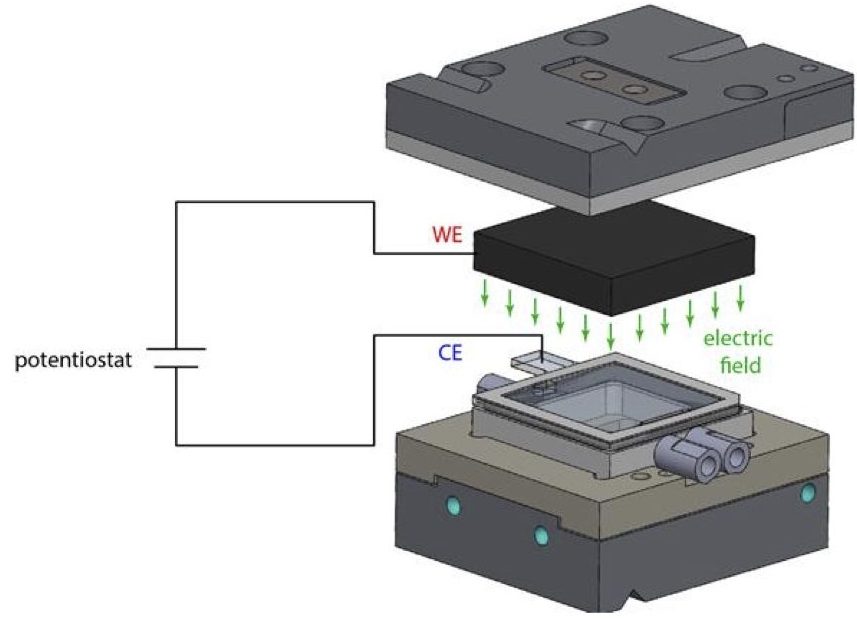
Figure 1. Schematic of home built electro-chemical cell for neutron reflectivity measurements.
Figure 2. Neutron reflectivity of (a) 5 and (b) 20% w/w [P6,6,6,14][BMB] in contrast matched propylene carbonate on a gold electrode at different applied potentials. The insets show SLD profiles obtained from model fits to the data, shown as solid lines in the main figures.
How?
A number of studies aimed to investigate the macro and nanofrictional properties of ILs have been conducted. It has been shown that ILs and their additives can facilitate significant reductions in friction, even when present in small concentrations in another base fluid. Furthermore, a limited number of studies have also demonstrated that the frictional properties of IL interfacial layers can be controlled “tribotronically” using an electric field. However, the mechanisms underlying and determining such phenomena are still not clear, particularly in the case of IL solutions. Neutron reflectivity (NR) is an excellent tool to study the structure and composition of buried interfaces. Using a home built electrochemical neutron reflectivity cell (cf. Fig.1) we have studied the electroresponsiveness of non-halogenated IL (trihexyl(tetradecyl)-phosphonium-bis(mandelato)borate, [P6,6,6,14][BMB]) at a gold electrode surface when dispersed in a base fluid (propylene carbonate) as a function of applied potential and IL concentration (cf. Fig 2). Upon varying the applied potential across the cell, we observe clear changes in the thickness and scattering length density of the IL interfacial layers next to the gold interface (cf. Fig. 2 insets). With increasing IL concentration, we also see that the IL layers become more densely packed and as a result extend less into the bulk than observed for the lower IL concentration.
Who?
This work was conducted by a team of researchers from KTH Royal Institute of Technology, Stockholm as part of the ongoing collaborative network “I-LEAP: Ionic liquids - lubrication enabling advanced performance” between KTH, Luleå University of Technology and Stockholm University. The NR measurements were conducted on the SuperADAM beamline at the Institute Laue-Langevin (ILL), France.
What's next?
We will continue study the structure of the non-halogenated IL ([P6,6,6,14][BMB]) at an electrified gold surface as a function of applied potential at different IL concentrations in propylene carbonate and as function of added water concentration. The results obtained from this study will help to fully elucidate the electro-structuring of ILs when dispersed in a base fluid, as well provide valuable complementary information on the influence of water at IL/electrode interfaces. The integration of such knowledge with complementary tribology tests is unique to our constellation and will help guide the application of ILs in future tribotronic systems, as well as provide a launch pad for other electrochemical applications where responsive interfacial structures are likely to play a key role
Contact: Dr Georgia Pilkington
georgiap@kth.se
KTH
The team is composed by Dr Anna Oleshkevych, Dr. Kathryn Harris, Akepati Bhaskar Reddy, Dr Seiya Watanabe, Dr Patricia Pedraz and Dr Milad Radiom, Prof. Mark Rutland and Prof. Sergei Glavatskih.
This project is funded by the Knut and Alice Wallenberg Foundation and the Swedish Research Council. The experimental data were collected on the Super ADAM beamline at the Institut Laue-Langevin (ILL), Grenoble, France. For further information about this research, and related projects, please visit www.ionicliquids.se.
