Why?
Lately the effect of global warming has been generally accepted and the interest of renewable resources is constantly increasing. Hydrogen would serve this purpose as an alternative fuel; however, storage is a challenging task and there is a need for new materials, with higher hydrogen capacity and lower cost.
The concept of high-entropy alloys (HEAs) was originally demonstrated 2004. This concept is based on equimolar alloys of five or more elements where a solid solution with a simple structure is formed. The solid solution is assumed to be thermodynamically stabilized by a high entropy of mixing, ΔSmix. Since 2004 the interest of HEAs has increased rapidly due to their promising mechanical properties for structural applications.
HEAs have also been studied as hydrogen storage materials, e.g. the HfNbTiVZr alloy has been demonstrated to possess excellent hydrogen storage properties. Specifically, it was shown that this alloy could be hydrogenated with a H/M ratio of at least 2.5 in a body centered tetragonal hydride phase, which is much more than in any other transition metal hydride. This is only possible if hydrogen could occupy unusual sites in the structure.
The purpose of this study was to investigate the mechanism of hydride formation in HfNbTiVZr. The temperature dependence and kinetics of the hydrogenation process was studied using in-situ synchrotron radiation powder X-ray diffraction (SR-PXD) combined with neutron powder diffraction (NPD) experiments. The latter enables determination of the position of the hydrogen atoms in the HEA hydride.
How?
Since the position of hydrogen in a metal matrix is very hard to detect using X-ray diffraction, neutron diffraction was needed to locate the hydrogen atoms in the structure. In-situ NPD was performed at the instrument D1B at Institute Laue-Langevin (ILL) in Grenoble, France. The sample was placed inside a steel tube and heated up to 500 °C at 50 bar D2 while measuring continuously. Additional measurements were performed at the PUS diffractometer at the JEEP II reactor at Institute for Energy Technology (IFE), Kjeller, Norway at ambient pressure and temperature. The figure below shows some of the important results of the published work [1].
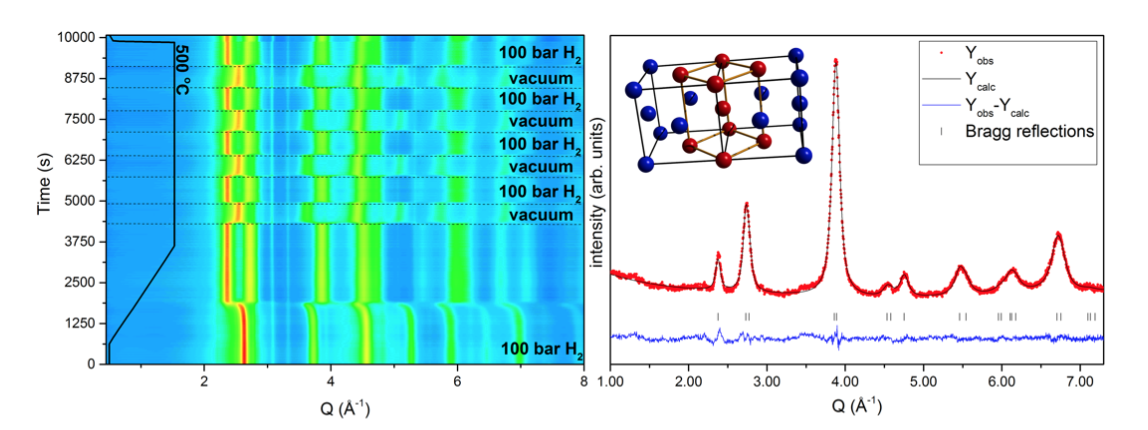
Left figure: In-situ SR-PXD of hydrogen cycling experiments at 500 oC. Right figure: Ex-situ NPD pattern at room temperature after obtaining the deuteride at 500 oC and 50 bar.
From the neutron diffraction measurements, the hydrogen was determined to occupy both the tetrahedral and octahedral interstitial sites in the tetragonal structure at high temperature and pressure (500 °C, 50 bar D2). These results are important to understand the unique properties of these alloys, and provides insight on how to develop them even further.
Who?
The project is led by Assoc. Prof Martin Sahlberg at the Department of Chemistry – Ångström Laboratory as Uppsala University, in collaboration with scientists from Norway, Denmark, Germany and France. The neutron diffraction experiments were performed at Institute Laue-Langevin (ILL) in Grenoble, France and at the JEEP II reactor at Institute for Energy Technology (IFE), Kjeller, Norway.
What is next?
We still need to learn more about how these high entropy materials react with hydrogen, and how this can be tuned using chemical modifications. Inelastic neutron scattering (INS) experiments are being performed at ISIS, UK. It is hoped that INS spectra will provide a clearer picture about the distribution of H atoms in tetrahedral and octahedral interstices and also give insight into the dynamic properties of these materials.
[1] D. Karlsson, G. Ek, J. Cedervall, C. Zlotea, K.T. Møller, T. C. Hansen, J. Bednarčík, M. Paskevicius, M.H. Sørby, T. R. Jensen, U. Jansson, M. Sahlberg. “Structure and hydrogenation properties of an HfNbTiVZr high-entropy alloy”, Inorganic Chemistry 2018 57 (4), 2103-2110
Contact:
Martin Häggblad Sahlberg.
