Plant superfood turned extraordinary surface glue: Neutrons reveal all
New research using the INTER reflectometer at ISIS Neutron and Muon Source has revealed how plant proteins can be used to stick particles to solid surfaces, opening up a plethora of potential industrial applications.
Professor Adrian Rennie (UU) –– An international collaboration of researchers from Sweden, Namibia and the UK have used neutron reflectivity to investigate the properties of a seed protein extracted from Moringa oleifera – a plant native to India. By studying the binding of 2S Albumin to various interfaces, scientists can determine how effective it is as a means of binding particles to interfaces in a controlled manner. The team hope that by doing so, they will open doors to future developments in the production of active filters for use in water treatment in remote locations. Their research may also help future scientists to develop methods to design and preserve nanostructures with potential applications in highly sensitive photonic devices.
If you scan the shelves of any healthcare store you will probably come across Moringa tea, oil or powder. This green substance resembles the more famous wheatgrass or barley grass powder that is often a key component of superfood smoothies. The use of Moringa trees is not limited to the leaves, seeds of these trees are a rich source of nutrition and oil which is used for cooking and health products. A member of the Moringaceae family, Moringa oleifera (more commonly known as the 'drumstick tree') grows in tropical and sub-tropical climates (25-35°C), requiring a net rainfall of between 250 and 3000mm per year. The plant is jam-packed full of nutrients, boasting a variety of essential phytochemicals. In fact, the nutritive properties of Moringa are so extensive that it is often referred to as the “tree of life", used to cure more than 300 types of disease.
Whilst the characteristic properties of Moringa lie within its leaves and seed oil – at least with regards to healthcare purposes – it is the seed proteins that a team of scientists led by Professor Adrian Rennie from Uppsala University were most interested in. Previously identified as effective flocculating agents for a whole host of impurities, Moringa seed proteins provide significant advantages over conventional industrially-produced materials such as cationic polymers of polyvalent salts. And that's not all. They boast low toxicity and a negligible environmental impact meaning that they are great candidates for flocculants in the context of water treatment processes. These special properties make them especially applicable to remote areas where there is a high demand for such technologies. Not only are they useful for filtering, but they also retain self-assembled structures like colloidal particles that might form at interfaces. By fixing designated nanostructures in a defined place, scientists can have greater control over their movement, which may well come in handy with regards to facilitating the low-cost technique manufacture of photonic devices.
Previous research identified that Moringa seed proteins were capable of adsorbing to a range of interfaces. We now know that a key component of the proteins found within these novel, natural flocculating agents is 2S albumin. The team extracted these proteins and then used a range of techniques - including neutron reflectivity - to investigate its use as a means to bind particles in a controlled manner at interfaces.
The characteristic properties of neutrons mean that they are especially effective for in-situ investigation of protein adsorption to surfaces and the binding of particles to protein layers. For this reason, neutron reflectometry (Figure 1) was the technique of choice employed by the scientists and what better instrument to use for this purpose than the INTER reflectometer, based at the ISIS Neutron and Muon Source. The way this works boils down to the way in which neutrons interact with the nuclei of atoms. Whilst the science behind this process is intrinsically detailed, the key message to take home is that neutrons are capable of penetrating deep into a sample with very few interactions. In fact, it is this high penetration depth that makes certain materials like Si02 'transparent' for neutrons – which is of course very handy when you want to study the interactions at the water/silica surface.
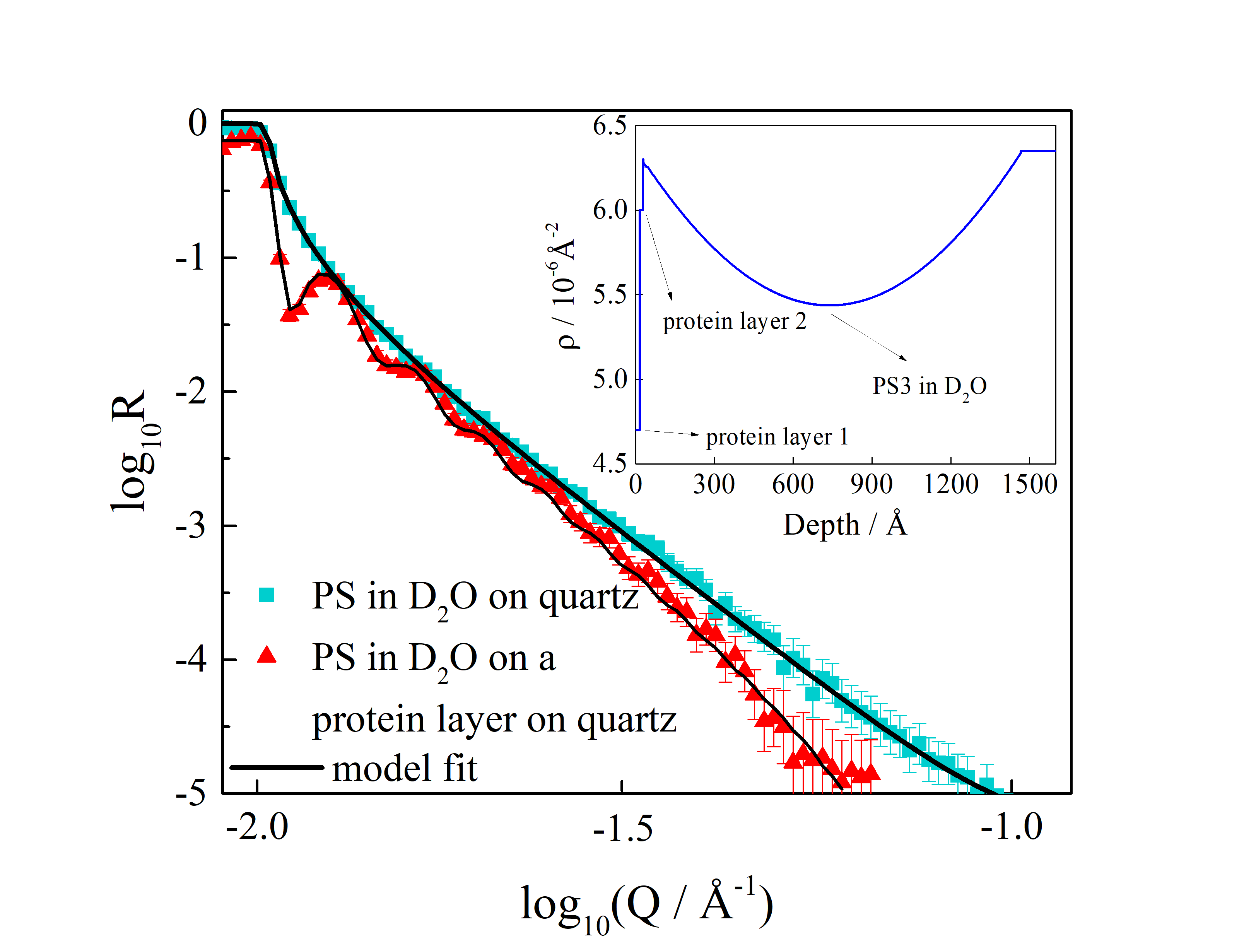
So what did the team discover? When they counted the particles that adhered to the water/silica surface from dilute dispersions, they recorded a sticking probability of about 1. Not only that, but once the particles had attached to the surface they couldn't be removed, either by rinsing with water or with a solution of cationic surfactant (C16TAB). Intriguingly, the atomic force microscopy (AFM) image of the sample following neutron experiments at ISIS suggested that there were regions of the silica/water interface that had a highly ordered coverage of particles. What these results mean is that scientists might be able to develop surfaces in the future that have been patterned with different materials in order to actually control adherence and create specific structures on those patterns.
Further information
The research was supported by the Swedish Research Council and the full publication is available to view in Colloids and Surfaces B: BiointerfacesRead the original article here. For further information about the research, please contact Professor Adrian Rennie (adrian.rennie@physics.uu.se)
To learn more about INTER and its capabilities, please follow this link.
Browse all of the science highlights from ISIS Neutron and Muon Source here.